⚗️HELP In the following acid-base reaction, NH4+ is the H2PO4- (aq) + NH3(aq) → HPO42- (aq) + - Brainly.com

SOLVED: which of the following is not a conjugate acid base pair?A) H3PO4 and HPO4^2-B) H2PO4^- and HPO4^2- C) HPO4^2- and PO4^3-D) H3PO4 and H2PO4^-E) H2O and H3O
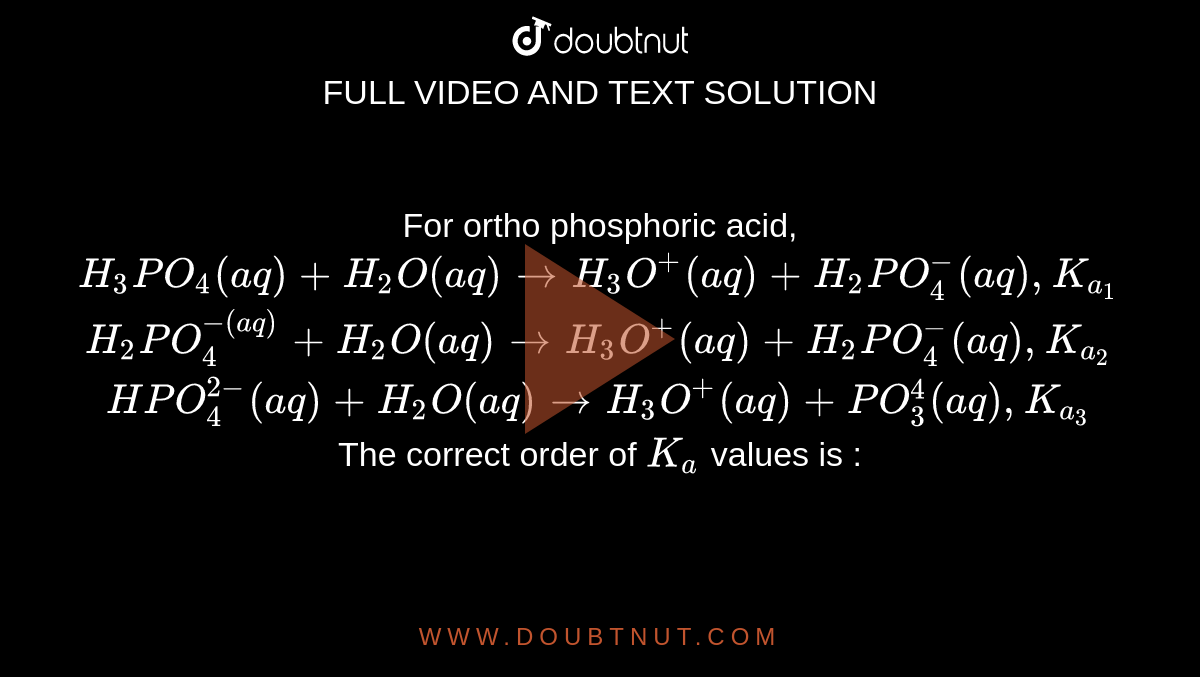
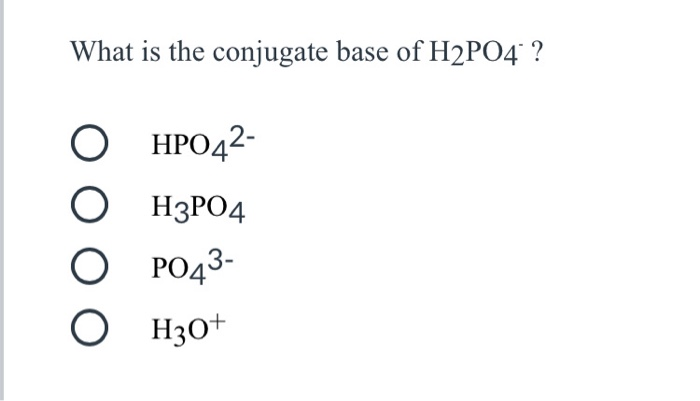
The dihydrogen phosphate ion undergoes these reaction in water H2PO4^(-)(aq)+H2O(l)toHPO4^(2-)(aq)+H3O^(+)(aq) K=6.2xx10^(-8) H2PO4^(-)(aq)+H2O(l)toH3PO4(aq)+OH^(-)(aq) K=1.6xx10^(-7) What is the conjugate base of H2PO4^(-) ?

Fillable Online UNIT 14 - Acids & Bases ACID BASE HSO4 H3PO4 NO3 H2PO4 ... Fax Email Print - pdfFiller

What is the conjugate base of H2PO4⁻? Which of the following is a WEAK acid? - Home Work Help - Learn CBSE Forum
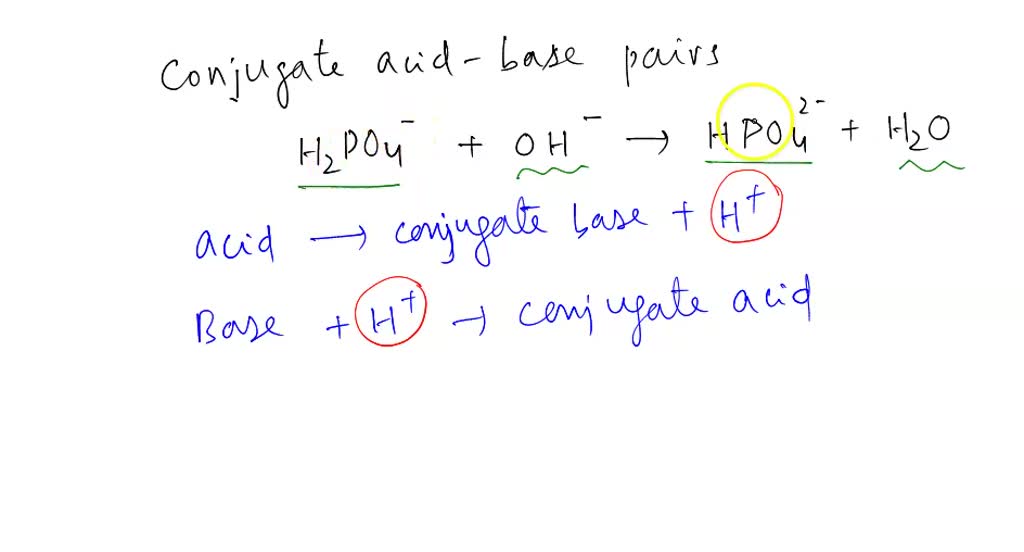
![ANSWERED] Which of the following are conjugate acid... - Physical Chemistry ANSWERED] Which of the following are conjugate acid... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/70938391-1657397871.813889.jpeg)